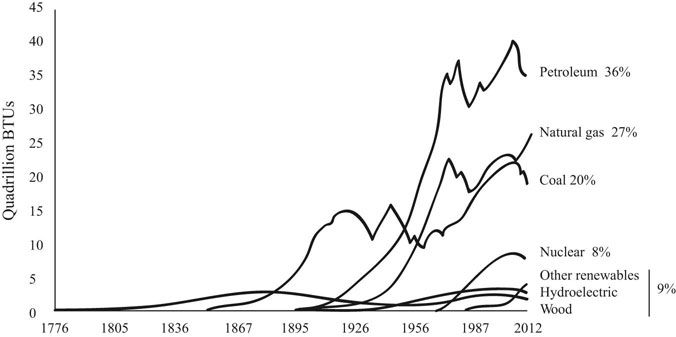
Wood was the primary energy source throughout most of human history and remained so up until the industrial revolution. Population increases in Europe reduced the forest cover from 95 percent during the Roman Empire to 20 percent at the start of the industrial revolution (Figure 31.1). The shortage of wood and the energy density of coal combined with the steam engine to usher in the age of fossil fuels (Gore 2009, 52).
Conventional fuels can be classified as solid fuel, coal, liquid fuel, oil, and oil byproducts, such as gasoline, and gaseous fuels, such as methane. Liquid fuels have very high energy content per volume and are easily moved through pipelines and tanker trucks to distribution points where vehicles can have their gas or oil tanks filled up. These characteristics make liquid fuels very useful for transportation. Oil is currently the fuel society uses the most (Gore 2009, 54). Natural gas is the next primary energy source after oil. Natural gas is used primarily in industry but also to heat homes. Natural gas is the cleanest of the conventional fuels because it has fewer impurities and it has a higher ratio of hydrogen to carbon (Gore 2009, 55). When a fuel is burned the fuel is oxidized. The atoms in the fuel are rearranged to a lower energy state with the accompanying release of heat energy. The energy comes from the rearrangement of the hydrogen (H) and the oxygen (O) atoms. Methane is CH4, and oxygen is O2.
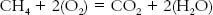
Sometimes there are other atoms in a fuel, such as sulfur and or nitrogen. Then the combustion process produces sulfur oxides and nitrous oxides, which create smog. These impurities can be significantly cleaned from the exhaust gases, but usually not completely so. An important concept to understand is that the complete combustion of a fuel produces carbon dioxide (CO2) and water (H2O). There is no way to combust a fuel without producing carbon dioxide.
FIGURE 31.1 Energy use in the United States by fuel source.
Source: United States Energy Administration, Annual Energy Review 2011. www.eia.gov/are.
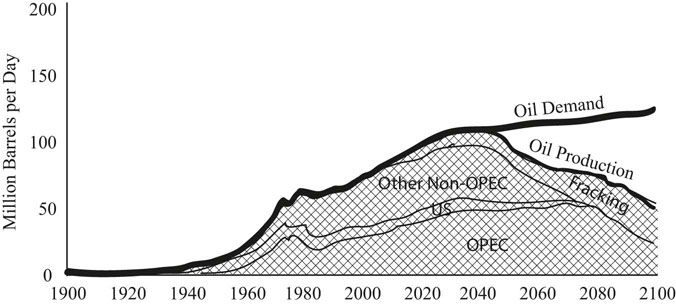
FIGURE 31.2 Projecting oil supply and demand through 2100.
Source: United States Energy Administration, Annual Energy Review 2011. www.eia.gov/are.
Burning natural gas results in 48 percent less carbon dioxide than burning coal, and 28 percent less carbon dioxide than burning oil (Gore 2009, 54–55). Natural gas produces less carbon dioxide per unit of energy because natural gas (CH4) has a higher hydrogen to carbon ratio than coal or oil. Energy comes from the rearranging of the hydrogen and oxygen atoms. The carbon is along for the ride. In addition, oil and coal have significantly more impurities in them, with the resulting need to take the impurities out before or after combustion.
A rapidly approaching problem with the world’s dependence on oil is that peak oil production will happen in the next few decades. Since demand for energy will continue to increase as the developing world expands, the price of the remaining dwindling oil supplies will increase quickly (Figure 31.2).
Electricity is a very convenient energy carrier. It is produced by all of the fuels mentioned above. Coal, oil, or gas is burned and uranium is split to create heat that produces steam that turns turbines to produce electricity. Electricity is very useful to provide light and turn motors, so demand has grown. The United States generates electricity using coal (42 percent), gas (25 percent), nuclear (19 percent), hydro (8 percent), and other and renewables (6 percent). (U.S. Energy Information Administration 2011, 222) The dependence on coal for electricity is a major carbon dioxide problem. Another issue with electricity generation is that only about 35 percent of the energy content of the fuel burned to produce the electricity ends up as electricity; 65 percent of the fuel energy goes up the stack as waste heat.
Total energy, or cogeneration, systems generate electricity and then use the waste heat to create hot water for heating with heat exchangers and chilled water for cooling with absorption chillers. This process is used in situations where there is a consistent electricity demand coincident with a nearby heating and or cooling demand. Cogeneration can approach using 90 percent of the energy content of the fuel used.
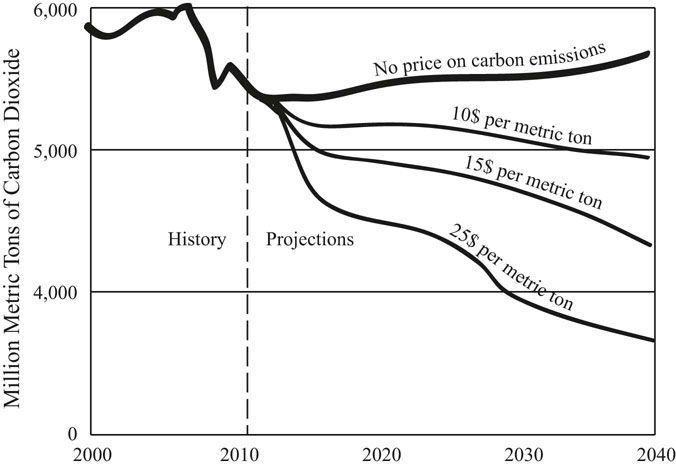
Taxing the use of fossil fuels through a carbon tax would have the dual benefit of raising revenue for the government and reducing the use of fossil fuels and thus carbon dioxide (Figure 31.4). A carbon tax would push users of coal toward natural gas, and in the long run cause a transition to wind, solar, and bio-fuels. The revenue generated by the tax could be used to facilitate the transition to alternative energy sources.
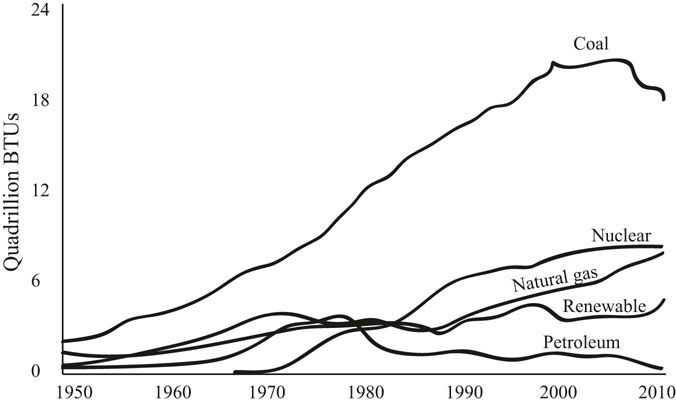
FIGURE 31.3 Electricity generation in the United States by fuel source.
Source: United States Energy Administration, Annual Energy Review 2011. www.eia.gov/are.
FIGURE 31.4 The reduction of carbon dioxide emissions resulting from a carbon tax of $10, $15, and $25 per metric ton of carbon dioxide.
Source: United States Energy Information Agency, Annual Energy Review 2013. www.eia.gov/are